1 min read
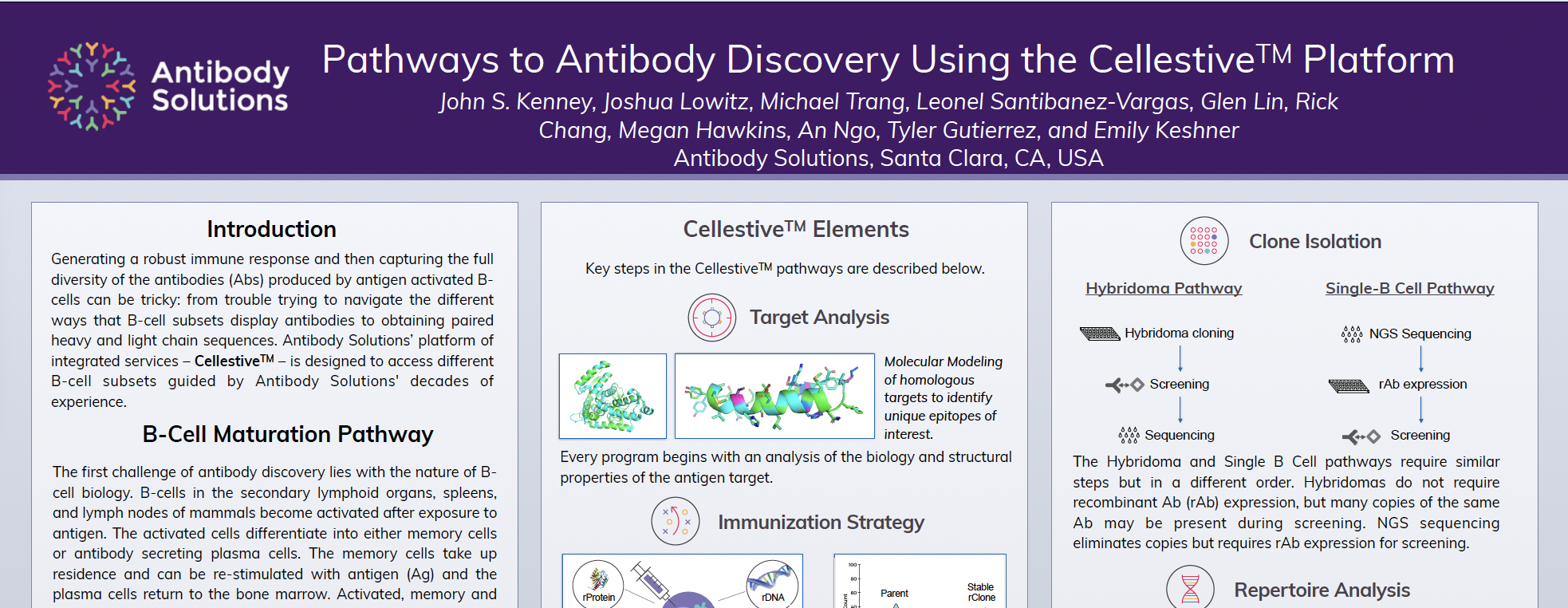
Pathways to Antibody Discovery Using the Cellestive™ Platform
By Antibody Solutions Research Team on Nov 14, 2023 7:13:43 AM
CellestiveTM leverages the success of immune rodents in raising diverse and developable Abs. It can interrogate and isolate different B-cell populations using the same well-established technology (i.e., FACS), further enabling the diversity of Abs obtained.
Topics: Posters
1 min read
Discovery and characterization of prolactin neutralizing monoclonal antibodies for the treatment of female-prevalent pain disorders
By Antibody Solutions Research Team on Oct 5, 2023 11:19:20 AM
Prolactin (PRL) has recently been demonstrated to elicit female-selective nociceptor sensitization and increase pain-like behaviors in female animals. Here we report the discovery and characterization of first-in-class, humanized PRL neutralizing monoclonal antibodies (PRL mAbs). We obtained two potent and selective PRL mAbs, PL 200,031 and PL 200,039. PL 200,031 was engineered as human IgG1 whereas PL 200,039 was reformatted as human IgG4. Both mAbs have sub-nanomolar affinity for human PRL (hPRL) and produce concentration-dependent and complete inhibition of hPRL signaling at the hPRL receptor (hPRLR). These two PRL mAbs are selective for hPRL as they do not inhibit other hPRLR agonists such as human growth hormone or placental lactogen. They also cross-react with non-human primate PRL but not with rodent PRL. Further, both mAbs show long clearance half-lives after intravenous administration in FcRn-humanized mice. Consistent with their isotypes, these mAbs only differ in binding affinities to Fcγ receptors, as expected by design. Finally, PL 200,019, the murine parental mAb of PL 200,031 and PL 200,039, fully blocked stress-induced and PRL-dependent pain behaviors in female PRL-humanized mice, thereby providing in vivo preclinical proof-of-efficacy for PRL mAbs in mechanisms relevant to pain in females.
Topics: Monoclonal Publications Prolactin
1 min read
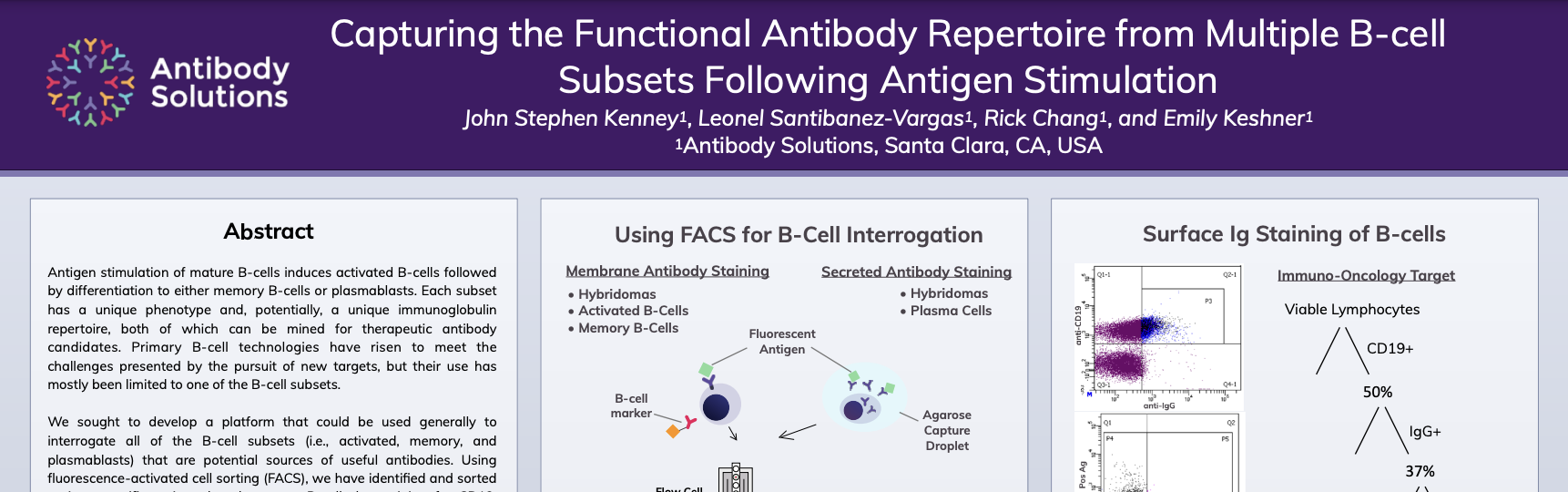
Capturing the Functional Antibody Repertoire from Multiple B-cell Subsets Following Antigen Stimulation
By Antibody Solutions Research Team on May 25, 2023 11:30:00 AM
B-cells are the original antibody display platform. Over time, they have proved to be the most reliable source of therapeutic antibodies. Capturing the full diversity of antibodies has been challenging due to how subsets of different b-cells display those antibodies and the ability to obtain paired heavy and light chain sequences. Our newly integrated platform of services – CellestiveTM – seeks to capture multiple functional B-cell subset repertoires using a flexible, comprehensive, and cost-effective approach.
Topics: Posters
1 min read
Antibodies Targeting Three Spike Protein Domains Regulating SARS-CoV-2 Infectivity
By Antibody Solutions Research Team on May 11, 2021 1:57:00 PM
Infectivity of SARS-CoV-2 is regulated by three sites on the spike protein: a receptor binding motif (RBM), a subunit (S)1/S2 cleavage site acted upon by the host protease furin, and an S2’ cleavage site acted upon by host protease TMPRSS2. The RBM of the spike protein binds to angiotensin converting enzyme 2 (ACE2) that is highly expressed in cells of the lower respiratory tract. Cleavage of the S1/S2 site by furin liberates a RRAR sequence that is C-terminal in the S1 domain that subsequently binds to neuropilin-1 receptors expressed in respiratory and olfactory epithelial cells. Additionally, cleavage at the S1/S2 site enables a structural change that allows further proteolytic processing by TMPRSS2 that is essential for membrane fusion.
Topics: SARS-CoV-2 Posters
1 min read
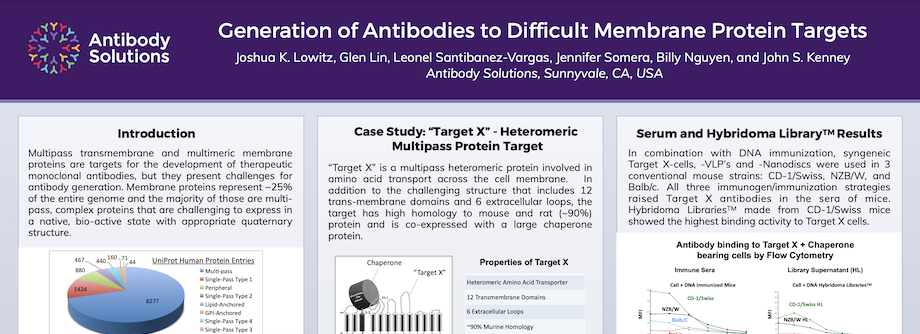
Generation of Antibodies to Difficult Membrane Protein Targets
By Antibody Solutions Research Team on Jan 5, 2021 1:24:00 PM
Multipass transmembrane and multimeric membrane proteins are targets for the development of therapeutic monoclonal antibodies, but they present challenges for antibody generation. Membrane proteins represent ~25% of the entire genome and the majority of those are multi-pass, complex proteins that are challenging to express in a native, bio-active state with appropriate quaternary structure.
Topics: Posters Multi-pass transmembrane Multi-meric Membrane Therapeutic Monoclonal Antibodies Antibody Generation
1 min read
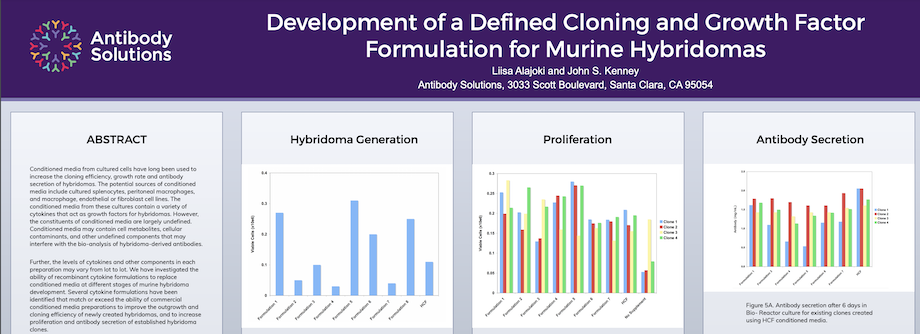
Development of a Defined Cloning and Growth Factor Formulation for Murine Hybridomas
By Antibody Solutions Research Team on Jan 5, 2021 12:50:00 PM
Conditioned media from cultured cells have long been used to increase the cloning efficiency, growth rate and antibody secretion of hybridomas. The potential sources of conditioned media include cultured splenocytes, peritoneal macrophages, and macrophage, endothelial or fibroblast cell lines. The conditioned media from these cultures contain a variety of cytokines that act as growth factors for hybridomas. However, the constituents of conditioned media are largely undefined. Conditioned media may contain cell metabolites, cellular contaminants, and other undefined components that may interfere with the bio-analysis of hybridoma-derived antibodies.
Topics: Posters Hybridoma Conditioned Media
1 min read
Antibody targeting functional domains of the SARS-CoV-2 Spike protein
By Antibody Solutions Research Team on Jan 4, 2021 10:17:00 AM
Infectivity of SARS-CoV-2 is regulated by three sites on the spike protein: a receptor binding motif (RBM), a subunit (S)1/S2 cleavage site acted upon by the host protease furin, and an S2’ cleavage site acted upon by host protease TMPRSS2. The RBM of the spike protein binds to angiotensin converting enzyme 2 (ACE2) that is highly expressed in cells of the lower respiratory tract. Cleavage of the S1/S2 site by furin liberates a RRAR sequence that is C-terminal in the S1 domain that subsequently binds to neuropilin-1 receptors expressed in respiratory and olfactory epithelial cells. Additionally, cleavage at the S1/S2 site enables a structural change that allows further proteolytic processing by TMPRSS2 that is essential for membrane fusion.
Topics: SARS-CoV-2 Posters
1 min read
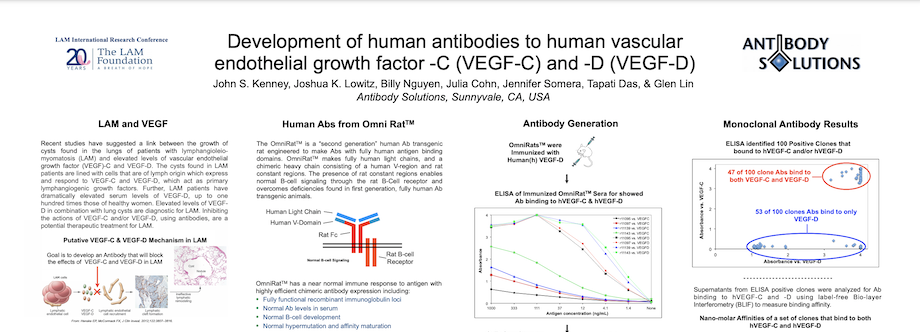
Development of human antibodies to human vascular endothelial growth factor -C (VEGF-C) and -D (VEGF-D)
By Antibody Solutions Research Team on Jan 1, 2020 1:49:00 PM
Recent studies have suggested a link between the growth of cysts found in the lungs of patients with lymphangioleiomyomatosis (LAM) and elevated levels of vascular endothelial growth factor (VEGF)-C and VEGF-D. The cysts found in LAM patients are lined with cells that are of lymph origin which express and respond to VEGF-C and VEGF-D, which act as primary lymphangiogenic growth factors. Further, LAM patients have dramatically elevated serum levels of VEGF-D, up to one hundred times those of healthy women. Elevated levels of VEGFD in combination with lung cysts are diagnostic for LAM. Inhibiting the actions of VEGF-C and/or VEGF-D, using antibodies, are a potential therapeutic treatment for LAM.
Topics: Posters VEGF-D Lymphangioleiomyomatosis LAM VEGF-C
1 min read
Generation Using a Molecular Modeling Platform to Guide Therapeutic Antibody Discovery
By Antibody Solutions Research Team on Dec 9, 2019 10:40:00 AM
"Using a Molecular Modeling Platform to Guide Therapeutic Antibody Discovery” details the integration of advanced 3-D modeling tools for the analysis of proteins and antibody-antigen interactions in our discovery platform. We show that adding molecular 3D modeling to the mix can reduce the cost and time of antibody discovery.
Topics: Transgenic Animals Posters Human Therapeutic Antibodies Immunization
1 min read
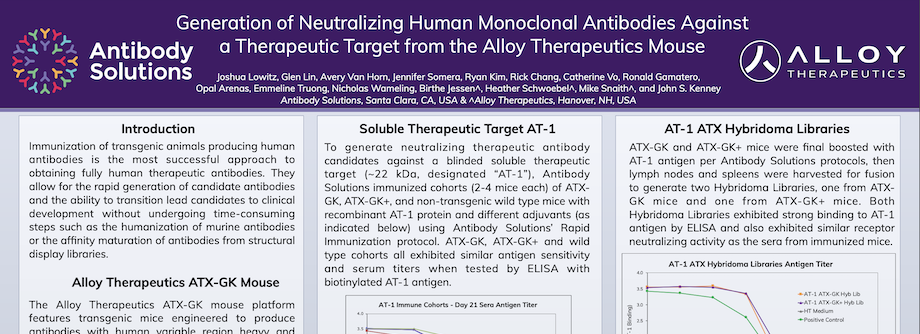
Generation of Neutralizing Human Monoclonal Antibodies Against a Therapeutic Target from the Alloy Therapeutics Mouse
By Antibody Solutions Research Team on Dec 9, 2019 10:31:00 AM
Immunization of transgenic animals producing human antibodies is the most successful approach to obtaining fully human therapeutic antibodies. They allow for the rapid generation of candidate antibodies and the ability to transition lead candidates to clinical development without undergoing time-consuming steps such as the humanization of murine antibodies or the affinity maturation of antibodies from structural display libraries.
Topics: Posters Monoclonal ATX-GX Alloy Therapeutics
1 min read
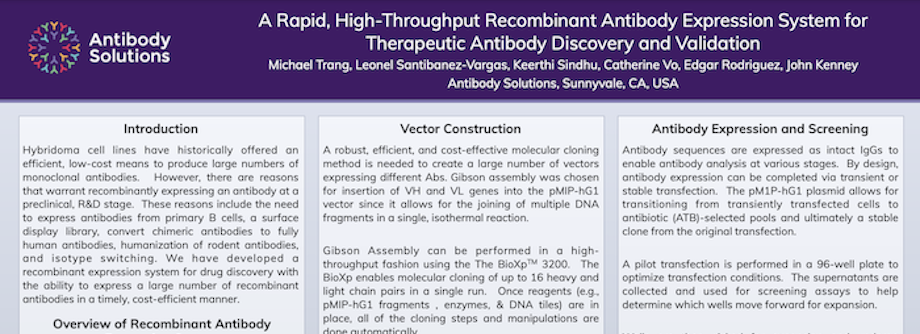
A Rapid, High-Throughput Recombinant Antibody Expression System for Therapeutic Antibody Discovery and Validation
By Antibody Solutions Research Team on Dec 1, 2018 10:13:00 AM
Hybridoma cell lines have historically offered an efficient, low-cost means to produce large numbers of monoclonal antibodies. However, there are reasons that warrant recombinantly expressing an antibody at a preclinical, R&D stage. These reasons include the need to express antibodies from primary B cells, a surface display library, convert chimeric antibodies to fully human antibodies, humanization of rodent antibodies, and isotype switching. We have developed a recombinant expression system for drug discovery with the ability to express a large number of recombinant antibodies in a timely, cost-efficient manner.
Topics: Posters Hybridoma Monoclonal
1 min read
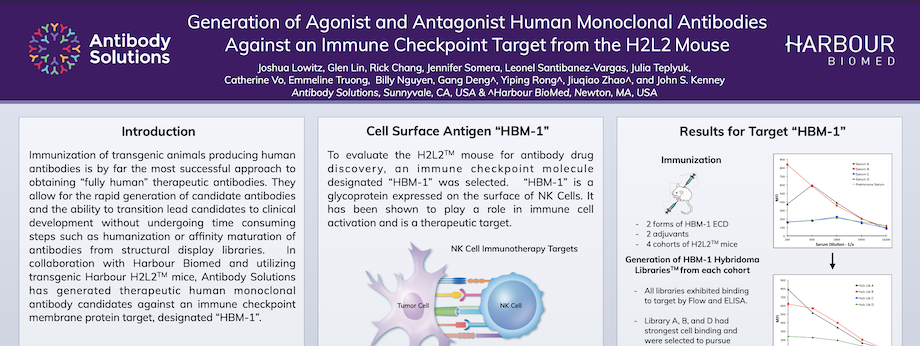
Generation of Agonist and Antagonist Human Monoclonal Antibodies Against an Immune Checkpoint Target from the H2L2 Mouse
By Antibody Solutions Research Team on Apr 30, 2018 12:38:00 PM
Immunization of transgenic animals producing human antibodies is by far the most successful approach to obtaining “fully human” therapeutic antibodies. They allow for the rapid generation of candidate antibodies and the ability to transition lead candidates to clinical development without undergoing time consuming steps such as humanization or affinity maturation of antibodies from structural display libraries. In collaboration with Harbour Biomed and utilizing transgenic Harbour H2L2TM mice, Antibody Solutions has generated therapeutic human monoclonal antibody candidates against an immune checkpoint membrane protein target, designated “HBM-1”.
Topics: Transgenic Animals Posters Fully Human
1 min read
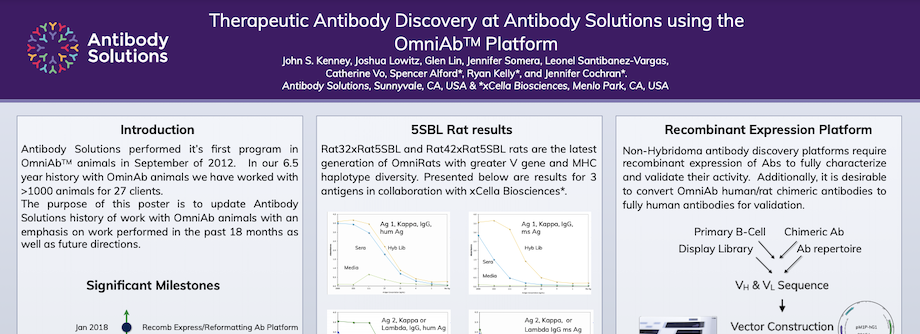
Therapeutic Antibody Discovery at Antibody Solutions using the OmniAbTM Platform
By Antibody Solutions Research Team on Apr 29, 2018 1:42:00 PM
Antibody Solutions performed its first program in OmniAbTM animals in September of 2012. In our 6.5 year history with OminAb animals we have worked with >1000 animals for 27 clients. The purpose of this poster is to update Antibody Solutions history of work with OmniAb animals with an emphasis on work performed in
Topics: Posters OmniAb
1 min read
Optimization of Therapeutic Discovery Strategies for Human Antibody Transgenic Animal Platforms
By Antibody Solutions Research Team on Apr 8, 2018 10:55:00 AM
Immunization of transgenic animals producing human antibodies has been the most successful approach to obtaining fully human therapeutic antibodies. They allow for the rapid generation of candidate antibodies to multiple targets of interest and the ability to transition lead candidates to clinical development without undergoing time consuming and costly steps such as humanization or affinity maturation of antibodies from structural display libraries.
Topics: Transgenic Animals Posters Human Therapeutic Antibodies
1 min read
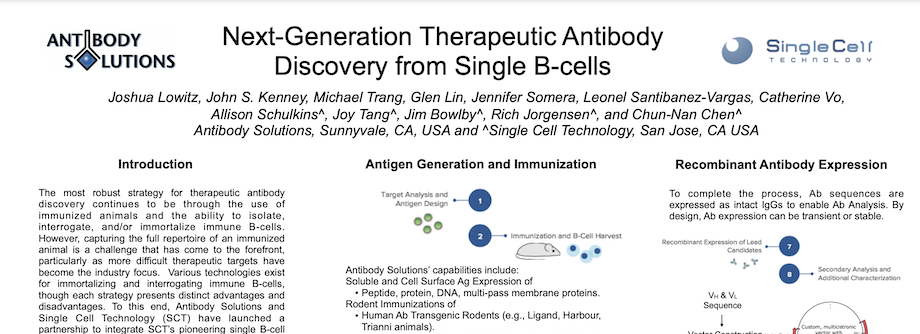
Next-Generation Therapeutic Antibody Discovery from Single B-cells
By Antibody Solutions Research Team on Jan 8, 2018 1:39:00 PM
The most robust strategy for therapeutic antibody discovery continues to be through the use of immunized animals and the ability to isolate, interrogate, and/or immortalize immune B-cells. However, capturing the full repertoire of an immunized animal is a challenge that has come to the forefront, particularly as more difficult therapeutic targets have become the industry focus. Various technologies exist for immortalizing and interrogating immune B-cells, though each strategy presents distinct advantages and disadvantages. To this end, Antibody Solutions and Single Cell Technology (SCT) have launched a partnership to integrate SCT’s pioneering single B-cell analysis technology with Antibody Solutions’ comprehensive antibody discovery platform.
Topics: Antibody Discovery Posters Immune B-cells
1 min read
Generation and Selection of Human Monoclonal Antibodies from the H2L2 Mouse
By Antibody Solutions Research Team on Jan 8, 2018 1:33:00 PM
Human antibody-producing transgenic H2L2TM mice have been used to generate monoclonal antibodies to therapeutic targets. Transgenic animals producing human antibodies are by far the most successful approach to obtaining “fully human” therapeutic antibodies. This is largely due to the ability to move transgenic animalderived human antibodies from lead selection directly to clinical development without undergoing lead optimization steps common with humanization of murine Abs or affinity maturation of Abs from phage or other synthetic libraries.
Topics: Posters Transgenic H2L2 Mice
1 min read
Development of Antibody and PK and ADA Assays for a Cystine Knot Fusion Protein
By Antibody Solutions Research Team on Jan 1, 2018 12:00:00 AM
Cystine knot peptides, also know as knottins, are an emerging class of biotherapeutic molecules that can be engineered to bind to a diverse range of targets. Much like therapeutic antibodies, there is a need to develop critical reagents for pharmacokinetic (PK) and immunogenicity assays to support preclinical development, though this new class of molecules can present different challenges and considerations for reagent discovery. To this end, monoclonal and polyclonal reagent antibodies against a cystine-knot fusion protein (CKFP) were generated and used for PK and ADA assay development.
Topics: Posters Knottins Cystine Knot Peptides
1 min read
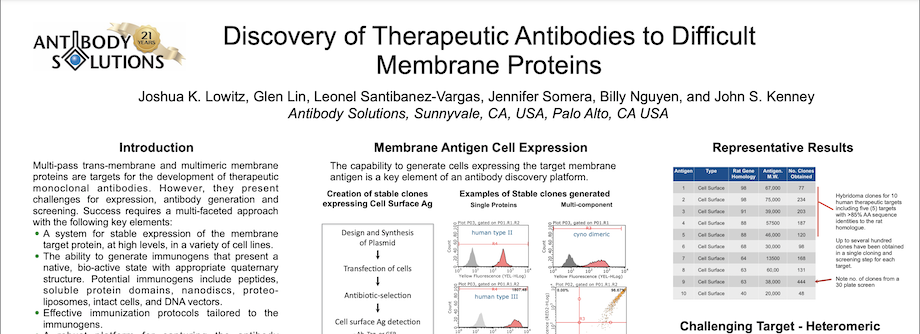
Discovery of Therapeutic Antibodies to Difficult Membrane Proteins
By Antibody Solutions Research Team on Dec 11, 2016 1:11:00 PM
Multi-pass trans-membrane and multimeric membrane proteins are targets for the development of therapeutic monoclonal antibodies. However, they present challenges for expression, antibody generation and screening. Success requires a multi-faceted approach with the following key elements:
- A system for stable expression of the membrane target protein, at high levels, in a variety of cell lines.
- The ability to generate immunogens that present a native, bio-active state with appropriate quaternary structure. Potential immunogens include peptides, soluble protein domains, nanodiscs, proteoliposomes, intact cells, and DNA vectors.
- Effective immunization protocols tailored to the immunogens.
- A robust platform for capturing the antibody response.
- A high-throughput screening platform for detecting antibodies on target protein-expressing cells
Topics: Posters Multi-pass transmembrane Multi-meric Membrane Therapeutic Monoclonal Antibodies
1 min read
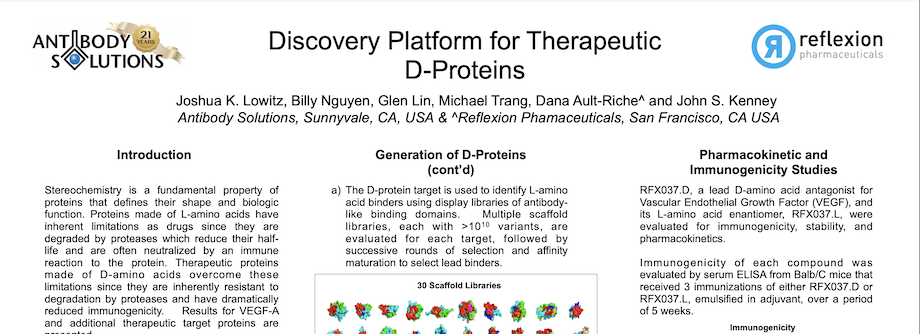
Discovery Platform for Therapeutic D-Proteins
By Antibody Solutions Research Team on Dec 11, 2016 12:55:00 PM
Stereochemistry is a fundamental property of proteins that defines their shape and biologic function. Proteins made of L-amino acids have inherent limitations as drugs since they are degraded by proteases which reduce their halflife and are often neutralized by an immune reaction to the protein. Therapeutic proteins made of D-amino acids overcome these limitations since they are inherently resistant to degradation by proteases and have dramatically reduced immunogenicity. Results for VEGF-A and additional therapeutic target proteins are presented
Topics: Posters Stereochemistry L-amino acids
1 min read
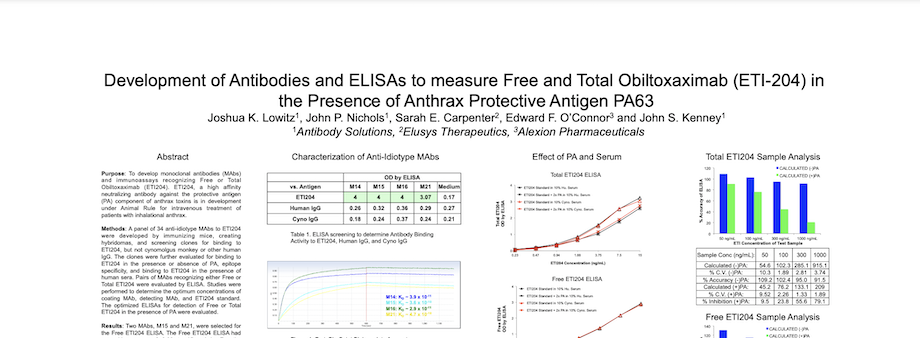
Development of Antibodies and ELISAs to measure Free and Total Obiltoxaximab (ETI-204) in the Presence of Anthrax Protective Antigen PA63
By Antibody Solutions Research Team on Jun 8, 2015 1:15:00 PM
Preclinical and clinical pharmacokinetic (PK) studies require highly specific and high affinity immunoassays to evaluate the safety and efficacy of therapeutic monoclonal antibodies (MAbs). In addition to the requirement that immunoassays specifically detect therapeutic MAbs without interference from host serum proteins, it is increasingly critical to develop reagents that allow differentiation between Free (Unbound) and Total (Bound + Unbound) drug. These reagents help to more fully characterize in vivo drug:target interaction and illustrate the full pharmacologic effect of therapeutic MAbs.
Topics: Posters Therapeutic Monoclonal Antibodies Pharmacokinetic (PK) ELISAs
1 min read
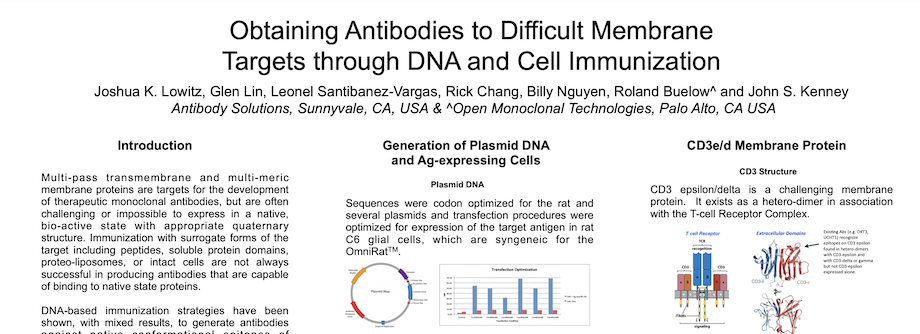
Obtaining Antibodies to Difficult Membrane Targets through DNA and Cell Immunization
By Antibody Solutions Research Team on Jan 5, 2015 10:22:00 AM
Multi-pass transmembrane and multi-meric membrane proteins are targets for the development of therapeutic monoclonal antibodies, but are often challenging or impossible to express in a native, bio-active state with appropriate quaternary structure. Immunization with surrogate forms of the target including peptides, soluble protein domains, proteo-liposomes, or intact cells are not always successful in producing antibodies that are capable of binding to native state proteins.
Topics: Posters Multi-pass transmembrane Multi-meric Membrane Therapeutic Monoclonal Antibodies
1 min read
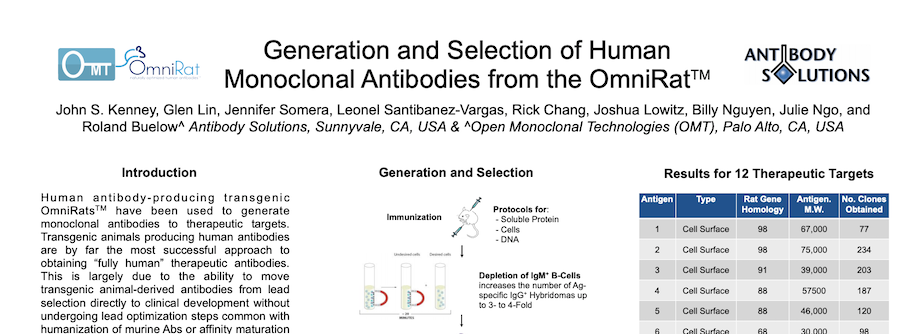
Generation and Selection of Human Monoclonal Antibodies from the OmniRat
By Antibody Solutions Research Team on May 5, 2014 1:51:00 PM
Human antibody-producing transgenic OmniRatsTM have been used to generate monoclonal antibodies to therapeutic targets. Transgenic animals producing human antibodies are by far the most successful approach to obtaining “fully human” therapeutic antibodies. This is largely due to the ability to move transgenic animal-derived antibodies from lead selection directly to clinical development without undergoing lead optimization steps common with humanization of murine Abs or affinity maturation of Abs from phage or other synthetic libraries.
Topics: Posters Therapeutic Targets OmniRat
2 min read
Chemical Synthesis and X-ray Structure of a Heterochiral {D-protein antagonist plus vascular endothelial growth factor} Protein Complex by Racemic Crystallography
By Antibody Solutions Research Team on Nov 11, 2012 11:28:00 AM
Total chemical synthesis was used to prepare the mirror image (D-protein) form of the angiogenic protein vascular endothelial growth factor (VEGF-A). Phage display against D-VEGF-A was used to screen designed libraries based on a unique small protein scaffold in order to identify a high affinity ligand. Chemically synthesized D- and L- forms of the protein ligand showed reciprocal chiral specificity in surface plasmon resonance binding experiments: The L-protein ligand bound only to D-VEGF-A, whereas the D-protein ligand bound only to L-VEGF-A. The D-protein ligand, but not the L-protein ligand, inhibited the binding of natural VEGF(165) to the VEGFR1 receptor. Racemic protein crystallography was used to determine the high resolution X-ray structure of the heterochiral complex consisting of {D-protein antagonist + L-protein form of VEGF-A}. Crystallization of a racemic mixture of these synthetic proteins in appropriate stoichiometry gave a racemic protein complex of more than 73 kDa containing six synthetic protein molecules. The structure of the complex was determined to a resolution of 1.6 Å. Detailed analysis of the interaction between the D-protein antagonist and the VEGF-A protein molecule showed that the binding interface comprised a contact surface area of approximately 800 Å(2) in accord with our design objectives, and that the D-protein antagonist binds to the same region of VEGF-A that interacts with VEGFR1-domain 2.
Topics: Publications VEGF-A D Protein
1 min read
Ligand Binding Assays in the 21st Century Laboratory: Recommendations for Characterization and Supply of Critical Reagents
By Antibody Solutions Research Team on Jun 14, 2012 11:27:00 AM
Critical reagents are essential components of ligand binding assays (LBAs) and are utilized throughout the process of drug discovery, development, and post-marketing monitoring. Successful lifecycle management of LBA critical reagents minimizes assay performance problems caused by declining reagent activity and can mitigate the risk of delays during preclinical and clinical studies. Proactive reagent management assures adequate supply. It also assures that the quality of critical reagents is appropriate and consistent for the intended LBA use throughout all stages of the drug development process. This manuscript summarizes the key considerations for the generation, production, characterization, qualification, documentation, and management of critical reagents in LBAs, with recommendations for antibodies (monoclonal and polyclonal), engineered proteins, peptides, and their conjugates. Recommendations are given for each reagent type on basic and optional characterization profiles, expiration dates and storage temperatures, and investment in a knowledge database system. These recommendations represent a consensus among the authors and should be used to assist bioanalytical laboratories in the implementation of a best practices program for critical reagent life cycle management.
Topics: Publications LBAs Critical Reagents
Cadherin-11 Antagonists and Methods for the Treatment of Inflammatory Joint Disorders
By Antibody Solutions Research Team on Jul 5, 2011 3:38:00 PM
The present invention relates to Cadherin-11 antagonists and compositions comprising Cadherin-11 antagonists. The invention also relates to methods for treating inflammatory joint disorders, such as rheumatoid arthritis, in a mammalian subject by administering a therapeutically effective amount of a Cadherin-11 antagonist.
Topics: Publications Cadherin-11
1 min read
Modulation of Lysyl Oxidase-like 2 Enzymatic Activity by an Allosteric Antibody Inhibitor
By Antibody Solutions Research Team on Jul 2, 2010 11:26:00 AM
In this report, we assessed the steady-state enzymatic activity of lysyl oxidase-like 2 (LOXL2) against the substrates 1,5-diaminopentane (DAP), spermine, and fibrillar type I collagen. We find that both DAP and spermine are capable of activating LOXL2 to the same extent and have similar Michaelis constants (K(m) approximately 1 mm) and catalytic rates (k(cat) approximately 0.02 s(-1)). We also show that LOXL2 is capable of being inhibited by a known suicide inhibitor of lysyl oxidase (LOX), beta-aminopropionitrile, which we find is a potent inhibitor of LOXL2 activity. The modality of inhibition of beta-aminopropionitrile was also examined and found to be competitive with respect to the substrates DAP and spermine. In addition, we identified an antibody inhibitor (AB0023) of LOXL2 enzymatic function and have found that the inhibition occurs in a non-competitive manner with respect to both spermine and DAP. The binding epitope of AB0023 was mapped to the scavenger receptor cysteine-rich domain four of human LOXL2. AB0023 binds to a region remote from the catalytic domain making AB0023 an allosteric inhibitor of LOXL2. This affords AB0023 several advantages, because it is specific for LOXL2 and inhibits the enzymatic function of LOXL2 in a non-competitive manner thereby allowing inhibition of LOXL2 regardless of substrate concentration. These results suggest that antibody allosteric modulators of enzymatic function represent a novel drug development strategy and, in the context of LOXL2, suggest that inhibitors such as these might be useful therapeutics in oncology, fibrosis, and inflammation.
Topics: Publications LOXL2
1 min read
DLL4 Blockade Inhibits Tumor Growth and Reduces Tumor-Initiating Cell Frequency
By Antibody Solutions Research Team on Aug 7, 2009 11:26:00 AM
Previous studies have shown that blocking DLL4 signaling reduced tumor growth by disrupting productive angiogenesis. We developed selective anti-human and anti-mouse DLL4 antibodies to dissect the mechanisms involved by analyzing the contributions of selectively targeting DLL4 in the tumor or in the host vasculature and stroma in xenograft models derived from primary human tumors. We found that each antibody inhibited tumor growth and that the combination of the two antibodies was more effective than either alone. Treatment with anti-human DLL4 inhibited the expression of Notch target genes and reduced proliferation of tumor cells. Furthermore, we found that specifically inhibiting human DLL4 in the tumor, either alone or in combination with the chemotherapeutic agent irinotecan, reduced cancer stem cell frequency, as shown by flow cytometric and in vivo tumorigenicity studies.
Topics: Publications DLL4
1 min read
Regulation of BRCA2 Gene Expression by the SLUG Repressor Protein in Human Breast Cells
By Antibody Solutions Research Team on Apr 29, 2005 11:23:00 AM
The expression of the breast cancer susceptibility protein BRCA2 is highly regulated in human breast, ovary, and pancreatic cells. BRCA2 is not expressed in the non-dividing cells, and expression is cell cycle stage-dependent and is elevated in the sporadic cancer cells. Mutational analysis of the upstream sequence of the human BRCA2 gene revealed an E2-box-containing silencer at the -701 to -921 position. The E2-box is essential for the cell-cycle stage-dependent activity of the silencer. We affinity-purified a 29-kDa silencer-binding protein (SBP) from the nuclear extracts of human breast cells BT-549 and MDA-MB-231. We explored whether the E2-box-binding repressor protein SLUG, which is of similar molecular size, is involved in the silencing process. Supershift assay with the purified SBP and anti-SLUG antibody revealed the identity of the SBP as SLUG. We found that silencer is inactive in the human breast cancer cells such as MDA-MB-468 and MCF-7 that do not express SLUG, further suggesting the involvement of SLUG in the BRCA2 gene silencing. Inducible expression of human SLUG in the dividing MDA-MB-468 cells reduced BRCA2 RNA levels with the activation of the silencer. Furthermore, small interfering RNA-mediated knockdown of SLUG mRNA in the BT-549 cells caused inhibition of the silencer function. Chromatin immunoprecipitation assays suggested that SLUG mediates its action by recruiting C-terminal-binding protein-1 (CtBP-1) and histone deacetylase-1 (HDAC-1) at the silencer E2-box. The general HDAC inhibitor, trichostatin A, inhibited the SLUG-mediated regulation of the silencer function. It thus appears that SLUG is a negative regulator for BRCA2 gene expression.
Topics: Publications BRCA2
1 min read
Therapeutic Targeting of Endothelial Ligands for L-selectin (PNAd) in a Sheep Model of Asthma
By Antibody Solutions Research Team on Mar 16, 2005 11:24:00 AM
The homing of lymphocytes to peripheral lymph nodes is initiated by an adhesive interaction between L-selectin on lymphocytes and PNAd, a set of sialomucins that are constitutively displayed on high endothelial venules of lymph nodes. PNAd is defined by monoclonal antibody MECA-79 that recognizes a sulfated oligosaccharide carried by the sialomucins. This epitope overlaps with 6-sulfo sialyl Lewis x, a recognition determinant for L-selectin. Previous work has shown that administration of a L-selectin monoclonal antibody blocks both late-phase airway responses and airway hyperresponsiveness in a sheep model of asthma. We show here that airway-associated lymphoid collections from lungs of allergic sheep exhibited PNAd(+) venules as detected by immunostaining with MECA-79. The same vessels also expressed a GlcNAc-6-O-sulfotransferase known as HEC-GlcNAc6ST, which is known to contribute to the formation of the MECA-79 epitope in high endothelial venules of mouse lymph nodes. Intravenous administration of MECA-79 to allergic sheep significantly blunted both the late-phase airway response and airway hyperresponsiveness induced by airway allergen challenge. Furthermore, MECA-79 inhibited the accumulation of all classes of leukocytes in bronchoalveolar lavage fluid. These findings represent the first demonstration that targeting of PNAd has therapeutic efficacy in an inflammatory disease.
Topics: Publications L-selectin PNAd
1 min read
HIV-1 Vif Blocks the Antiviral Activity of APOBEC3G by Impairing Both its Translation and Intracellular Stability
By Antibody Solutions Research Team on Sep 12, 2003 11:22:00 AM
The human immunodeficiency virus type 1 (HIV-1) relies on Vif (viral infectivity factor) to overcome the potent antiviral function of APOBEC3G (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G, also known as CEM15). Using an APOBEC3G-specific antiserum, we now show that Vif prevents virion incorporation of endogenous APOBEC3G by effectively depleting the intracellular levels of this enzyme in HIV-1-infected T cells. Vif achieves this depletion by both impairing the translation of APOBEC3G mRNA and accelerating the posttranslational degradation of the APOBEC3G protein by the 26S proteasome. Vif physically interacts with APOBEC3G, and expression of Vif alone in the absence of other HIV-1 proteins is sufficient to cause depletion of APOBEC3G. These findings highlight how the bimodal translational and posttranslational inhibitory effects of Vif on APOBEC3G combine to markedly suppress the expression of this potent antiviral enzyme in virally infected cells, thereby effectively curtailing the incorporation of APOBEC3G into newly formed HIV-1 virions.
Topics: Publications HIV-1 CEM15 APOBEC3G
1 min read
ARMER, Apoptotic Regulator in the Membrane of the Endoplasmic Reticulum, a Novel Inhibitor of Apoptosis
By Antibody Solutions Research Team on May 11, 2003 11:21:00 AM
We have identified a novel protein, apoptotic regulator in the membrane of the endoplasmic reticulum (ARMER), which protects HT1080 fibrosarcoma cells from apoptosis induced by various stimuli. We demonstrate that ARMER is an endoplasmic reticulum (ER) integral membrane protein with four predicted transmembrane domains and a COOH-terminal KKXX ER retrieval motif. We used an inducible expression system (pIND) to study the effects of regulated ARMER overexpression. Cells in which ARMER was overexpressed exhibited protection from multiple apoptotic inducers including serum starvation, doxorubicin, UV irradiation, tumor necrosis factor alpha, and the ER stressors brefeldin A, tunicamycin, and thapsigargin. Analysis of the caspase proteolytic cascade reveals that ARMER inhibits proteolysis of the caspase-9-specific fluorogenic substrate LEHD-AFC as well as endogenous substrates downstream of caspase-9; however, it does not inhibit cytochrome c release or cleavage of caspase-9 itself. Apoptotic stimuli cause endogenous levels of ARMER protein and RNA to decrease, leading to cell death; however, sustaining ARMER protein levels through exogenous expression inhibits apoptosis. These data suggest that ARMER is a novel ER integral membrane protein which protects cells by inhibiting caspase-9 activity and reveal a possible role for ARMER in cell survival.
Topics: Publications ARMER
1 min read
Drosophila p53 is a Structural and Functional Homolog of the Tumor Suppressor p53
By Antibody Solutions Research Team on Mar 31, 2000 3:41:00 PM
The importance of p53 in carcinogenesis stems from its central role in inducing cell cycle arrest or apoptosis in response to cellular stresses. We have identified a Drosophila homolog of p53 ("Dmp53"). Like mammalian p53, Dmp53 binds specifically to human p53 binding sites, and overexpression of Dmp53 induces apoptosis. Importantly, inhibition of Dmp53 function renders cells resistant to X ray-induced apoptosis, suggesting that Dmp53 is required for the apoptotic response to DNA damage. Unlike mammalian p53, Dmp53 appears unable to induce a G1 cell cycle block when overexpressed, and inhibition of Dmp53 activity does not affect X ray-induced cell cycle arrest. These data reveal an ancestral proapoptotic function for p53 and identify Drosophila as an ideal model system for elucidating the p53 apoptotic pathway(s) induced by DNA damage.
Topics: Publications p53 Carcinogenesis
1 min read
Production of Monoclonal Antibodies Using a Secretion Capture Report Web
By Antibody Solutions Research Team on Aug 13, 1995 11:29:00 AM
We describe a novel method for the production of monoclonal antibodies using a secretion capture report web (SCRW). Following HAT selection in bulk culture, individual hybridomas are encapsulated in biotinylated agarose drops. Antibody secreted by the hybridoma is captured within the agarose drop using an avidin bridge and biotinylated anti-mouse immunoglobulin. The secreted antibody is detected by a fluorescent reporter which can be either a second anti-mouse antibody or an antigen. The binding of the reporter can be quantitated and the desired hybridoma directly cloned by flow cytometry. Multiparameter (i.e., two-color) reporter analysis can also be used to selectively enrich and clone rare hybridomas secreting antibodies directed to unique epitopes. The method allows the characterization of thousands of clones per second and the isolation of hundreds of clones per day.
Topics: Publications Secretion Capture Report Web
1 min read
Laboratory Methods in Immunology - Development of quantitative two-site ELISAs for soluble proteins.
By Antibody Solutions Research Team on May 23, 1990 11:17:00 AM
This two-volume reference details immunological techniques for biologists of all disciplines. Volume I includes a detailed discussion of the tissue culture laboratory. It addresses what the lab needs to be, and the general "housekeeping" procedures involved in tissue culture. Presented next are chapters on specific aspects of tissue culture and hybridoma technology. The book includes a review of bioassays for interleukins, and a series of papers on lymphokines and functional assays in vitro. The section on molecular genetic studies begins with consideration of the choice of strategies for cloning the genes of cell surface molecules. It continues with papers on aspects of molecular biological techniques most closely related to immunology. The final section covers immunochemical techniques. Volume II reviews techniques used with small laboratory animals. It includes papers on specialized procedures with animals. Technical aspects are emphasized through a detailed analysis of effects of ultraviolet light on the immune system. Covered also is antigen detection in cells and tissue. The book addresses the important areas of protein purification using monoclonal antibodies.These two volumes are of great importance to those who use immunological techniques, whether they are immunologists or trained in other disciplines. The book is intended for those in animal science, veterinary science, genetics and cell biology, bacteriology, immunology, pathology, biochemistry, laboratory medicine, and hematology.
Topics: ELISAs Publications
1 min read
Laboratory Methods in Immunology
By Antibody Solutions Research Team on May 9, 1990 11:00:00 AM
This two-volume reference details immunological techniques for biologists of all disciplines. Volume I includes a detailed discussion of the tissue culture laboratory. It addresses what the lab needs to be, and the general "housekeeping" procedures involved in tissue culture. Presented next are chapters on specific aspects of tissue culture and hybridoma technology. The book includes a review of bioassays for interleukins, and a series of papers on lymphokines and functional assays in vitro. The section on molecular genetic studies begins with consideration of the choice of strategies for cloning the genes of cell surface molecules. It continues with papers on aspects of molecular biological techniques most closely related to immunology. The final section covers immunochemical techniques. Volume II reviews techniques used with small laboratory animals. It includes papers on specialized procedures with animals. Technical aspects are emphasized through a detailed analysis of effects of ultraviolet light on the immune system. Covered also is antigen detection in cells and tissue. The book addresses the important areas of protein purification using monoclonal antibodies.These two volumes are of great importance to those who use immunological techniques, whether they are immunologists or trained in other disciplines. The book is intended for those in animal science, veterinary science, genetics and cell biology, bacteriology, immunology, pathology, biochemistry, laboratory medicine, and hematology.
Topics: Publications Tissue Culture
1 min read
Influence of Adjuvants on the Quantity, Affinity, Isotype and Epitope Specificity of Murine Antibodies
By Antibody Solutions Research Team on Jul 6, 1989 3:40:00 PM
Five adjuvants were compared to Freund's adjuvant for production of mouse polyclonal antibodies and monoclonal antibodies (McAbs) to human serum albumin (HSA) and interleukin-1 alpha (IL-1 alpha). Parameters examined were titer, affinity, concentration, isotype, epitope specificity and neutralizing activity of sera and hybridoma supernatants. Freund's adjuvant, while producing high titers and concentrations of antibodies in sera, was inferior to other adjuvants for eliciting antibodies with particular qualities. The adjuvants Quil A and A1(OH)3/[Thr1]muramyldipeptide elicited the highest affinity antibodies to HSA. Syntex adjuvant formulation-1 (SAF-1) elicited the highest percentage of 'protective' IgG2a antibodies
Topics: Publications McAbs HSA IL-1 alpha
Filter by Keyword
- Posters (21)
- Publications (15)
- Therapeutic Monoclonal Antibodies (4)
- Monoclonal (3)
- Multi-meric Membrane (3)
- Multi-pass transmembrane (3)
- Transgenic Animals (3)
- ELISAs (2)
- Human Therapeutic Antibodies (2)
- Hybridoma (2)
- SARS-CoV-2 (2)
- APOBEC3G (1)
- ARMER (1)
- ATX-GX (1)
- Alloy Therapeutics (1)
- Antibody Discovery (1)
- Antibody Generation (1)
- BRCA2 (1)
- CEM15 (1)
- Cadherin-11 (1)
- Carcinogenesis (1)
- Conditioned Media (1)
- Critical Reagents (1)
- Cystine Knot Peptides (1)
- D Protein (1)
- DLL4 (1)
- Fully Human (1)
- HIV-1 (1)
- HSA (1)
- IL-1 alpha (1)
- Immune B-cells (1)
- Immunization (1)
- Knottins (1)
- L-amino acids (1)
- L-selectin (1)
- LAM (1)
- LBAs (1)
- LOXL2 (1)
- Lymphangioleiomyomatosis (1)
- McAbs (1)
- OmniAb (1)
- OmniRat (1)
- PNAd (1)
- Pharmacokinetic (PK) (1)
- Prolactin (1)
- Secretion Capture Report Web (1)
- Stereochemistry (1)
- Therapeutic Targets (1)
- Tissue Culture (1)
- Transgenic H2L2 Mice (1)
- VEGF-A (1)
- VEGF-C (1)
- VEGF-D (1)
- p53 (1)