5 min read
Peptides: The Less-is-More Strategy for Success
A strategic approach to peptide design yields success
When deciding which antigens to use for an antibody discovery project, there are many available options. Peptides, which have been around for at least 60 years, are one way in. Other — and, in many cases, newer — available paths include recombinant proteins, engineered cells and DNA coding for the protein.
Typically, these provide a diverse population of epitopes or potential antibody recognition sites. Compared with the pathways listed above, a peptide program tends to have less epitopic coverage than the other immunogen choices that present longer or complete portions of the protein of interest. Despite being a foundational pathway in Antibody Discovery, it may seem that peptides don't offer the same diversity as other antigen options.
So what does your project have to gain from using peptides? Quite a lot, actually. Truth is, peptides offer a “less-is-more” approach that can enhance your discovery process.
-
They often lack immunodominant portions that may conceal the site of interest.
-
They can generate the fine specificity needed if your goal is to focus on a specific location or domain on the antigen.
-
They can bring to the fore an epitope whose sequence is either highly homologous or non-homologous between species.
And from a purely practical perspective, peptides also have the advantage of being easy, quick and inexpensive to synthesize in comparison to other forms of antigens.
The keys to designing the right peptides
Peptides have long been the antigen of choice for developing antibodies for immunohistochemistry and Western Blotting. These types of assays work under denatured conditions, so short linear peptides can generate antibodies that will work well in such applications.
However, in applications requiring the recognition of natively-folded structure, not just any peptide will do. Peptides can be especially challenging for raising antibodies to conformational epitopes that have a secondary structure or that are composed of discontinuous (or distant) sequences that are proximal in the natively folded target antigen. These considerations must be addressed to discover antibodies that will work as therapeutic antibodies or in ELISA, flow cytometry and blocking assays. These cases all require a peptide which sufficiently mimics the native protein structure.
In our design process, we use known and predicted structures of the target antigen and practical considerations. The process of evaluating which peptide is best suited for the job usually involves checking the target against an online database (e.g., UniProt), comparing for cross-reactivity between different species and predicting secondary structure.
In addition, we often capitalize on our in-house 3-D modeling software here at Antibody Solutions to predict the secondary and tertiary structures of various peptide candidates, as well as to compare that structure to that of the native protein. We perform this analysis because both length and amino acid sequence can affect the secondary structure of the peptide. In some instances, a designer can mimic a structural feature of the target by constructing disulfide linkages.
The peptide must have good water solubility and a moiety for conjugation to a carrier protein. For solubility, the designed peptides require a sufficient number of hydrophilic residues. Most commonly, we will conjugate the peptide to a carrier protein away from the most immunogenic end of the peptide. The carrier proteins most often used at Antibody Solutions are Bovine Serum Albumin (BSA) and Ovalbumin (OVA). The classic carrier protein, Keyhole Limpet Hemocyanin (KLH), while itself highly immunogenic, may not be the best carrier protein for raising anti-peptide antibodies and may generate off-target antibodies. Conjugations to different carrier proteins may be an option; one conjugated peptide might be used for immunizations, while the other peptide (conjugated to a different carrier protein) can be used for screening assays.
Case Study: A successful peptide design for the SARS-CoV-2 Spike protein
In 2020, Antibody Solutions designed peptide immunogens to target three functional sites of the SARS-CoV-2 Spike protein applying the knowledge and techniques outlined above. Our team relied on known and predicted structures along with molecular modeling to create a total of six peptides spanning the functional domains of the Spike protein. We were able to successfully raise antibodies to each of these domains that bind to native spike proteins.
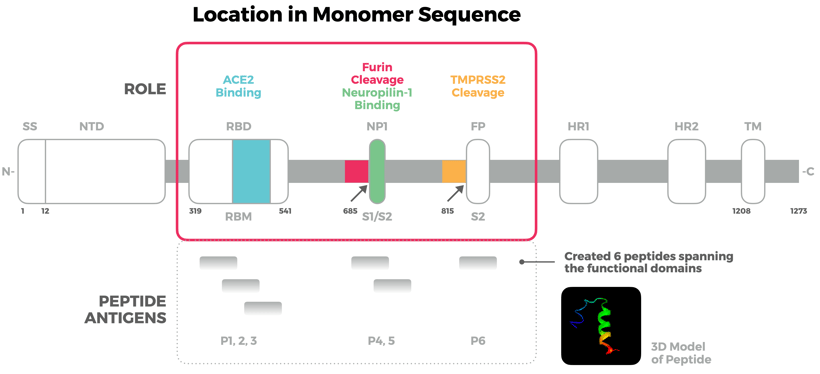
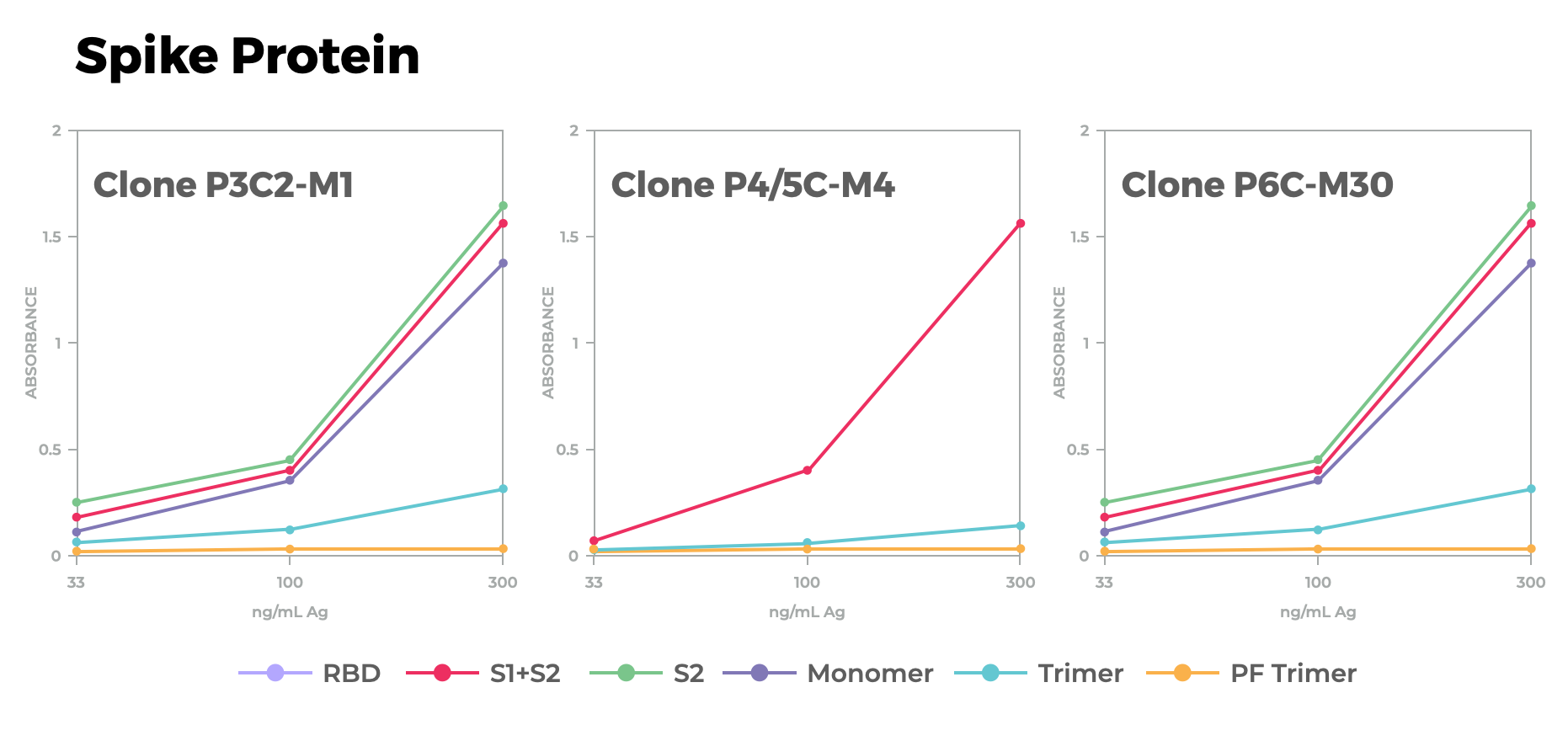
Figure 1. Spike protein binding of a selected clone antibody raised using peptide. This figure, derived from our poster, shows a linear representation of the SARS-CoV-2 Spike protein monomer. Outlined in red are the three domains we pursued that regulate virus infectivity. The middle of the figure shows the location of the six peptides that we designed as immunogens in relation to those areas of interest on the Spike protein — three for the RBM, two for the S1/S2 site, and one for the S2’ site.). Also pictured is a 3-D model of one of the peptides showing preserved secondary structure. The bottom of the figure shows binding by ELISA to spike protein variants by a selected clone raised to the domain-derived peptides.
You can read the full poster for “Antibody targeting functional domains of the SARS-CoV-2 Spike protein” here.
Hitting your goal
Antibody Solutions’ end goal is, of course, ushering the client’s novel therapy through the discovery process. Our customers rely on our more than two decades of innovative leadership in the field as well as an agile, transparent service platform for a reliable discovery process. Helping a client get to their target, however, means working both strategically and tactically with the science at our disposal. And there may be no better example of Antibody Solutions’ ability to achieve this than in peptide design.
We certainly enjoy talking about the science that supports our process, but what we enjoy the most is talking with you about your needs and interests. Whether you have a specific target antibody in mind or would simply like to know more about our approach, please don’t hesitate to reach out.
.png?width=80&height=80&name=Untitled%20design%20(18).png)
As one of our project managers, Jennifer works with customers in navigating their antibody discovery programs. Previously, she worked as a supervisor in the Cell Biology department at Antibody Solutions for 10 years. She has extensive experience in generating and culturing hybridomas, producing monoclonal antibodies and FACS sorting.




