1 min read
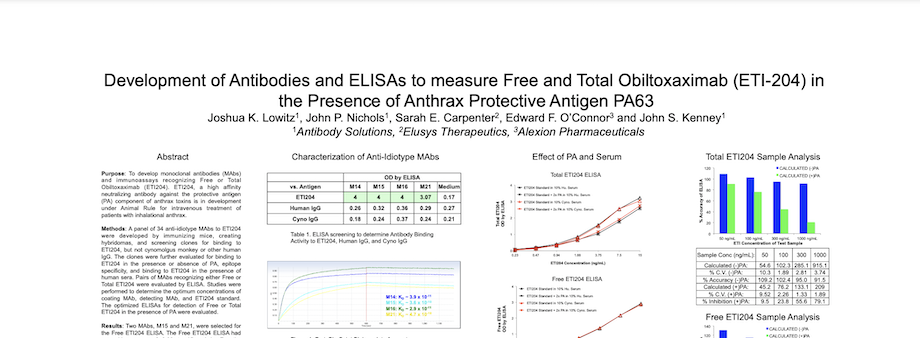
Development of Antibodies and ELISAs to measure Free and Total Obiltoxaximab (ETI-204) in the Presence of Anthrax Protective Antigen PA63
By Antibody Solutions Research Team on Jun 8, 2015 1:15:00 PM
Preclinical and clinical pharmacokinetic (PK) studies require highly specific and high affinity immunoassays to evaluate the safety and efficacy of therapeutic monoclonal antibodies (MAbs). In addition to the requirement that immunoassays specifically detect therapeutic MAbs without interference from host serum proteins, it is increasingly critical to develop reagents that allow differentiation between Free (Unbound) and Total (Bound + Unbound) drug. These reagents help to more fully characterize in vivo drug:target interaction and illustrate the full pharmacologic effect of therapeutic MAbs.
Topics: Posters Therapeutic Monoclonal Antibodies Pharmacokinetic (PK) ELISAs
Filter by Keyword
- Posters (21)
- Publications (15)
- Therapeutic Monoclonal Antibodies (4)
- Monoclonal (3)
- Multi-meric Membrane (3)
- Multi-pass transmembrane (3)
- Transgenic Animals (3)
- ELISAs (2)
- Human Therapeutic Antibodies (2)
- Hybridoma (2)
- SARS-CoV-2 (2)
- APOBEC3G (1)
- ARMER (1)
- ATX-GX (1)
- Alloy Therapeutics (1)
- Antibody Discovery (1)
- Antibody Generation (1)
- BRCA2 (1)
- CEM15 (1)
- Cadherin-11 (1)
- Carcinogenesis (1)
- Conditioned Media (1)
- Critical Reagents (1)
- Cystine Knot Peptides (1)
- D Protein (1)
- DLL4 (1)
- Fully Human (1)
- HIV-1 (1)
- HSA (1)
- IL-1 alpha (1)
- Immune B-cells (1)
- Immunization (1)
- Knottins (1)
- L-amino acids (1)
- L-selectin (1)
- LAM (1)
- LBAs (1)
- LOXL2 (1)
- Lymphangioleiomyomatosis (1)
- McAbs (1)
- OmniAb (1)
- OmniRat (1)
- PNAd (1)
- Pharmacokinetic (PK) (1)
- Prolactin (1)
- Secretion Capture Report Web (1)
- Stereochemistry (1)
- Therapeutic Targets (1)
- Tissue Culture (1)
- Transgenic H2L2 Mice (1)
- VEGF-A (1)
- VEGF-C (1)
- VEGF-D (1)
- p53 (1)